Solid Phase Peptide Synthesis Resins and Linkers - Omizzur

This article is a popular science guideline about peptide synthesis resins and linkers, with about 1800 words. You will be able to find all the knowledge about solid phase peptides synthesis resins and linkers here. Also you can mark this page for future use. Omizzur is a company focus on custom peptide synthesis, providing professional peptide services to global pharmaceutical R&D companies.
Peptides have a wide range of biological activities and potential applications, making peptide synthesis of great significance in fields such as drug development, biological research, and bioengineering. Peptides are molecules composed of multiple amino acids connected by peptide bonds, typically containing 10-100 amino acids. Peptide synthesis is a key technique in organic synthesis used to prepare peptide molecules with specific sequences and structures.
Basic Principles of Peptide Synthesis
Peptide bond formation: Peptide synthesis is accomplished through the formation of peptide bonds between amino acid residues. The formation of peptide bonds is achieved through the condensation reaction between the carboxyl and amino groups of amino acids.
Protective group strategy: Due to the varying reactivity of different functional groups in amino acids, it is necessary to use protective groups to protect specific functional groups from side reactions during the reaction.
Activation method: In order to improve the efficiency and selectivity of the reaction, amino acids need to be activated, usually using activators or coupling reagents.
The raw materials for peptide synthesis include various protected amino acids such as fmoc amino acids, resins, coupling agent, linkers, etc. This article will mainly introduce the advantages and disadvantages of various resins and the corresponding use of linking agents.
Main Methods for Peptide Synthesis
1. Solid phase peptide synthesis (SPPS)
Solid phase peptide synthesis is the most common chemical synthesis method, invented by chemist Bruce Merrifield in 1963. The solid-phase synthesis method greatly improves the efficiency of peptide synthesis. The basic principle is:
Using solid-phase resin as a support, fix the first amino acid onto the resin through covalent bonding. Then, by sequentially adding protected amino acid units, they are gradually connected into a polypeptide chain. After each synthesis step, the protective groups are removed through chemical treatment before proceeding to the next reaction step. Finally, the synthesized peptide was separated from the solid-phase resin.
The advantage of this method is that it is easy to automate and can produce peptides quickly and on a large scale. And the purification process is simple, without the need to separate intermediate products at every step.
2. Liquid phase peptide synthesis (LPPS)
Liquid phase peptide synthesis is an early method of peptide synthesis. Synthesis is carried out in solution, usually requiring separation and purification of each intermediate product between two steps. This method can be used to synthesize special peptides or peptides that require precise control. In some cases, it is suitable for producing longer and more complex peptide chains.
Solid Phase Peptide Synthesis Resins
Resin is one of the commonly used carrier materials in solid-phase peptide synthesis, which functions to tightly bind peptide chains and other organic molecules together, facilitating the reaction. Peptide synthesis emphasizes the chemical synthesis process of peptide molecules, and resin is a carrier material in the chemical synthesis process.
Firstly let's learn some important parameters about resins: crosslinking degree, mesh size, degree of substitution, and swelling degree:
1. Crosslinking degree: Taking PS resin as an example, its resin balls are formed by adding a certain amount of crosslinking agent divinylbenzene to polystyrene through polymerization reaction. The amount of crosslinking agent divinylbenzene added is the crosslinking degree (DVB), and the optimal crosslinking degree for the resin is 1%.
Higher crosslinking degree leads to a decrease in resin swelling degree, while lower crosslinking degree reduces the mechanical stability of the resin in the swollen state. Generally speaking, most commercially available resins have a crosslinking degree of 1%.
2. Mesh size: The size of the resin balls is generally 100-200 mesh (average diameter of 75-150 μ m) for commercially available resins, and the conditions of the polymerization reaction can affect the size of the resin balls.
3. Substitution degree: How many millimoles of active reaction sites are loaded per gram of resin, in mmol/g. It is worth noting that after obtaining the Wang resin and CTC resin commonly available on the market, we will load the first amino acid in the laboratory. However, the amino acid loading rate of these two resins cannot reach 100%, so the degree of substitution should be re measured after that.
4. Swelling degree: The swelling ability of resin in different solvents, measured in ml/g. Generally, after obtaining commercially available resins, we need to use solvents for swelling before use. Good swelling can increase the chance of reactants entering the site. DCM, DMF, and N-methylpyrrolidone are commonly used benign resin swelling solvents.
Introduction to Several Commonly Used Resins
1. Wang Resin
The resin commonly used in solid-phase peptide synthesis has a hydroxyl functional group, and the first amino acid is connected to the resin's linker through an ester bond. Therefore, the coupling conditions are more severe than amide bonds and require DMAP catalysis.
Due to the strong alkalinity of DMAP, amino acids (Cys, His) that are particularly prone to racemization are not suitable for loading onto Wang resin. In this case, CTC resin is recommended. The linker of Wang resin is relatively stable and requires high concentration TFA cleavage.
Wang Resin is an organic polymer resin with strong adsorption capacity, high selectivity, and stability. It can efficiently separate specific components in complex mixtures and achieve precise capture and fixation on its surface, providing an ideal solution for environmental protection and resource recovery.
Fmoc-Amino Acids attached to Wang Resin
| Fmoc-Ala-Wang resin | Fmoc-Val-Wang resin | Fmoc-Ile-Wang resin | Fmoc-Tyr(tBu)-Wang resin |
| Fmoc-Arg(Pbf)-Wang resin | Fmoc-Gln(Trt)-Wang resin | Fmoc-Leu-Wang resin | Fmoc-Pro-Wang resin |
| Fmoc-Asn(Trt)-Wang resin | Fmoc-Glu(OtBu)-Wang resin | Fmoc-Lys(Boc)-Wang resin | Fmoc-Ser(tBu)-Wang resin |
| Fmoc-Asp(OtBu)-Wang resin | Fmoc-Gly-Wang resin | Fmoc-Met-Wang resin | Fmoc-Thr(tBu)-Wang resin |
| Fmoc-Cys(Acm)-Wang resin | Fmoc-His(Trt)-Wang resin | Fmoc-Phe-Wang resin | Fmoc-Trp(Boc)-Wang resin |
2-Chlororityl Chloride Resin
2-Chlororityl Chloride Resin is a resin commonly used in solid-phase synthesis. The main feature of this resin is the presence of chlorinated aromatic groups on it, typically a 2-chlorotrityl structure, which provides reaction sites.
In solid-phase synthesis, chlorinated resin undergoes a substitution reaction with amino acids to fix them on the resin, allowing for the subsequent peptide synthesis steps. Through this method, each amino acid can be sequentially added to the polypeptide chain.
2-Chlororityl Chloride Resin is commonly used for the synthesis of shorter peptide chains, as longer chains may have lower reaction efficiency on such resins. This resin is of certain importance in chemical synthesis because it provides stable solid-phase support, which facilitates the gradual assembly of peptides.
| H-Ala-2-Chlorotrityl Resin | H-Gln-2-Chlorotrityl Resin | H-Met-2-Chlorotrityl Resin | H-Thr(Trt)-2-Chlorotrityl Resin |
| H-Arg(Pbf)-2-Chlorotrityl Resin | H-His(Trt)-2-Chlorotrityl Resin | H-Phe-2-Chlorotrityl Resin | H-Trp-2-Chlorotrityl Resin |
| H-Asn(Trt)-2-Chlorotrityl Resin | H-Ile-2-Chlorotrityl Resin | H-Pro-2-Chlorotrityl Resin | H-Tyr(tBu)-2-Chlorotrityl Resin |
| H-Asp(OtBu)-2-Chlorotrityl Resin | H-Leu-2-Chlorotrityl Resin | H-Ser(tBu)-2-Chlorotrityl Resin | H-Tyr(Trt)-2-Chlorotrityl Resin |
| H-Cys(Acm)-2-Chlorotrityl Resin | H-Lys(Boc)-2-Chlorotrityl Resin | H-Thr(tBu)-2-Chlorotrityl Resin | H-Val-2-Chlorotrityl Resin |
3. CTC resin
The resin commonly used in solid-phase peptide synthesis is extremely sensitive to water. When loading the first amino acid, there is no need for pre activation. Simply add the solution of amino acid and DIPEA to the resin to react, thus completely avoiding the racemic side reaction that occurs when Wang resin loads the first amino acid. Meanwhile, due to the site effect of chlorine atoms on the linker of CTC resin, it can effectively suppress the side reactions of DKP.
The linker of CTC resin is unstable, and low concentrations of TFA can cause it to cleave. Therefore, during the synthesis process, some peptide chains may detach from the resin, resulting in a decrease in yield. However, precisely because of this characteristic, we can use low concentrations of TFA to cleave peptide resins to obtain fully protected side chain multi peptide fragments for peptide fragment synthesis.
4. Rink amide-resin
It is the most commonly used resin for preparing peptide amides, with amino functional groups. Usually, we use Rink amide resin with amino groups protected by Fmoc. When loading the first amino acid, Fmoc should be removed first, and then enter the normal coupling washing deprotection cycle.
The linker of Rink amide resin is relatively stable and requires high concentration TFA cleavage, which can also produce side reactions similar to Wang resin.
Rink Amide resin is commonly used for terminal modification of peptide chains, which facilitates the connection of amino acids to the C-terminus of the peptide chain. It has good efficiency and selectivity in peptide synthesis, and is suitable for various solid-phase synthesis reactions.
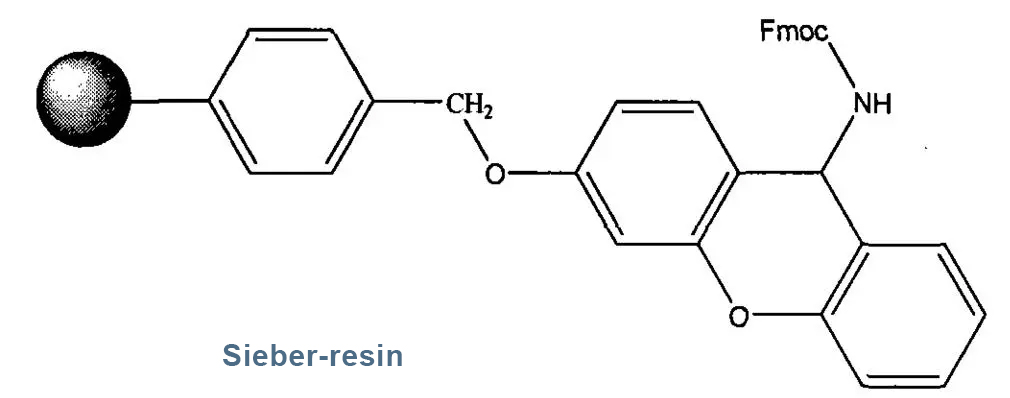
5. Sieber-resin
Sieber resin is a resin used for preparing peptide amides and is relatively expensive. Like Rink amide resin, the functional group is an amino group.
Usually, we use Sieber resin with amino groups protected by Fmoc. When loading the first amino acid, Fmoc should be removed first, and then enter the normal coupling washing deprotection cycle. The difference is that the linker of Sieber resin is not as stable as Rink amide resin, and low concentration TFA can be used to cleave peptide resin to obtain C-terminal acylated peptide fragments with fully protected side chains.
Omizzur also Provide other Popular Resins in Solid Phase Peptide Synthesis:
| Polystyrene Resin | Knorr Resin | Oxime Resin |
| Aminomethyl Polystyrene Resin | Knorr-2-Chlorotrityl Resin | PAM Resin |
| DHP HM Resin | MBHA Resin | Rink amide-MBHA Resin |
| HMPA-AM Resin | Merrifield Resin | Weinreb AM Resin |
Omizzur Linkers for Solid Phase Peptide Synthesis:
| Name | Purity(HPLC) | CAS |
| DHP Linker | 99% min. | 3749-36-8 |
| HMBA Linker | / | 3006-96-0 |
| HMP Linker | 99% min. | 68858-21-9 |
| Rink Amide Linker | 98.5% min | 145069-56-3 |
| Ramage Linker | 98%min | 212783-75-0 |
| Sieber Linker | 99% min | 3722-51-8 |
| Weinreb Linker | 99% min. | N/A |
Footnotes:
1:Bodanszky M. Principles of Peptide Synthesis. Berlin: Sringer-Verlag; 1984:158–201.
2:Field GB, Noble RL. Int J Pept Prot Res. 1990;35:161–214.
3:Albericio F, Kneib-Cordonier N, Biancalana S, et al. J Org Chem. 1990;55:3730–3743.
Read Related Articles:
Copyright © 2020 Omizzur Inc | Terms & Conditions | Privacy Notice | Sitemap