Peptide Solubility Guideline

Introduction: In the process of production and purification of peptide, insoluble sequences are often encountered, which also leads to the results of low yield, difficult purification, unqualified purity of the finished product or peptide production errors in many peptide enterprises.
These insoluble properties have a lot to do with the amino acid composition and sequence of the peptide itself. We have a long and in-depth study in the field of peptides, so that we have accumulated a wealth of excellent experience. Many customers do not know how to dissolve peptides when receiving products, how to choose the right solvent, resulting in reagent waste.
When attempting to dissolve a peptide in a solution that performs the biological function of the peptide, solubility becomes a focus of concern. All peptides can be dissolved in organic solvents. But this was not of much help because most peptides could not be studied in organic solvents. keyword: peptide solubility, peptide R&D
The Peptide Solubility Depends on Its Sequence
The solubility of a peptide ultimately depends on its sequence. pH is probably the most important in the conditions under which it can be operated. Omizur biotech has found that in many cases, a change of 1-3 pH units can completely dissolve a very stable peptide.
The transition curve is usually steep. In a very narrow pH range, the solution changes from cloudy to clear. This may be due to changes in the ionization state of some residues, such as His.
There is another reason why pH adjustment is important. Since HPLC solvents used for peptide purification contain 0.1% TFA that cannot be completely removed by lyophilization, received peptides usually contain small amounts of TFA. Which makes the peptide solution more acidic than you'd expect.
For peptides that are difficult to dissolve, you can prepare a higher concentration stock solution, such as DMS, in an organic solvent and dilute the peptide during biofunctional testing. Most tests can contain 1-2% of DSMO, some as much as 5%. But that doesn't always work. Some peptides are pooled and precipitated when diluted from DMSO and even at lower concentrations.
How to Find the Ideal Peptide Solubility Concentration
Finding the ideal peptide solubility can pose a serious challenge for researchers, as incorrect solubilization can lead to peptide loss or experimental failure.
Although many peptides have good solubility in aqueous solutions, some peptides have low solubility and insolubility. When peptides have long hydrophobic amino acid sequences, this poses a challenge to their dissolution. The following guidelines provide some suggestions to help predict the solubility of peptides.
Three Basic Principles to Choose the Right Solvent to Dissolve Peptides
Before use, customers can follow the following three basic principles to choose the right solvent to dissolve peptides. First, the solvent chosen must be able to dissolve the peptide adequately. Secondly, the selected solvent can be compatible with the experimental conditions, and finally, the selected solvent cannot react with the peptide, nor can it degrade the peptide. As long as the amount of peptide permits, a small part of the peptide can be dissolved first, and then the entire sample can be dissolved. If the peptide needs to be recovered from the solvent, an initial solvent can be selected.
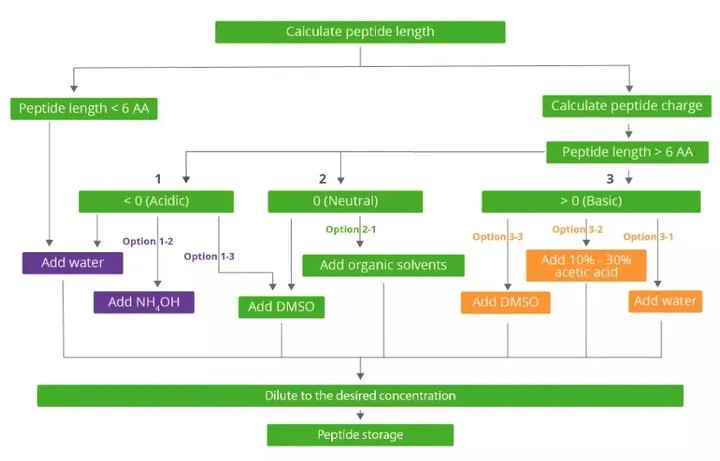
(Peptide Solubility Workflow)
The Following Suggestions May Help You Dissolve Peptides
1. Peptides with less than 5 amino acids are generally soluble in aqueous solutions, with the exception of strongly hydrophobic amino acids (W, I, L, F, M, V, Y).
2. If charged amino acids are evenly distributed throughout the sequence, then hydrophilic peptides containing > 25% charged amino acids (E, D, K, R, H) and peptides containing < 25% hydrophobic amino acids are generally soluble in aqueous solutions. The purification systems for peptides are generally 0.1%TFA/ water and 0.1%TFA/CAN.
Therefore, if the peptide is dissolved in a buffer with no or very weak buffer capacity, the result may be acidic peptide solutions. When using other methods to dissolve peptides, make sure that the pH of the solvent is nearly neutral. Acidic peptides (more E+D residue than K+R+H residue) and basic peptides (more K+R+H residue than E+D residue) are more soluble at neutral pH than at acidic pH.
3. Peptides containing 50% to 75% hydrophobic amino acids, even if the sequence contains 25% charged amino acids, may be insoluble in water or partially dissolved.
It is best to dissolve the peptide in the least amount of strong solvents (such as DMF, ACN, isopropyl alcohol, ethanol, acetic acid, 4-8M GdnHCl or urea, DMSO (when no C, W, M in the sequence), and other similar organic solvents, and it is best to slowly add the organic solvent to the aqueous solution drop by drop.
If the solution becomes cloudy, it may have reached its dissolution limit, and further dissolution is of little use. It is important to note that the initial solvent selected should be compatible with the experimental system.
4. Strong hydrophobic peptides with hydrophobic amino acid content > 75% are generally insoluble in aqueous solutions. Such peptides should also be dissolved first with a strong solvent (e.g., TFA, formic acid) and may also precipitate if added to an aqueous solution.
The final peptide solution may require a high concentration of organic solvents to denature it, and organic solvents are generally not used for biological function studies in living cells.
The peptide sequence with high content of S, T, E, D, K, R, H, N, Q, Y (> 75%) is easy to form redundant intermolecular hydrogen bond network, and it is easy to form gel in concentrated aqueous solution.
In order to reduce the possible problems in dissolution, it is recommended to consider the design and sequence to improve the solubility of the peptide.
Method of Dissolution of Peptide
Which solvent should be used to dissolve synthetic peptides has always been a difficult problem for peptide workers. Depending on the amino acid sequence, the following methods can be used to dissolve synthetic peptides. It is best to dissolve a small amount of the peptide in an aqueous solution first, rather than dissolve all the sample at once.
1. Combine the acid amino acids Asp (D), Glu (E) and C-terminal -COOH. The value is set to -1.
2. The values of basic amino acids Arg (R), Lys (K) and His (H) were set as +1.
3. Calculate the total amount of charge in the peptide.
4. If the total charge of the peptide is positive, the peptide is alkaline and can be dissolved with water first. If the peptide is insoluble in water, it can be dissolved with 10% acetic acid or higher. If the peptide is not soluble at all, add TFA(<50 ul) to help dissolve and dilute to 1ml with deionized water.
5. If the total charge of the peptide is negative, the peptide is acidic and can be dissolved with water first. If the peptide is insoluble in water, add NH4OH(<50 ul) to help dissolve and dilute to 1 ml with deionized water.
6. If the total charge of the peptide is 0, the peptide is neutral. Neutral peptides need to be dissolved with acetonitrile, methanol, isopropyl alcohol and other organic solvents, and denaturants (such as urea, guanidine hydrochloride, etc.) can also be added.
It is important to note that the solubility of peptides is a complex problem and is influenced by many factors. Accurate prediction of the solubility of peptides may require a comprehensive analysis combining experimental data and computational methods. Bioinformatics tools and predictive models can provide initial information, but the final judgment should be made under experimental conditions.
Read Related Articles:
Copyright © 2020 Omizzur Inc | Terms & Conditions | Privacy Notice | Sitemap