Structure impurities in synthetic peptide medicines
peptides are the products of dehydration and condensation of amino acids.According to the U. S. Pharmacopeia definition, the condensation product with the number of amino acids under 40 is called a peptide;The condensation product with more than 40 amino acids needs to consider its spatial structure, which is called protein. peptides are easy to be synthesized and sequence optimized, and their medicinal value can be determined quickly,Because of their nature character, peptides take much less time to get FDA approval from clinical trials than small-molecule drugs, which are twice as likely to pass clinical trials as small-molecule compounds. Compared with macromolecular proteins or antibody drugs, peptides are more stable with less dosage and higher unit activity.Compared with macromolecular protein, peptide chemical synthesis technology is more mature, easy to be separated from impurities and with higher purity.
The chemical synthesis of peptides begins with the liquid phase synthesis, but each step of the synthesis of peptides in the liquid phase has to be purified and separated, and the operation is complicated. In 1963, American chemist Merrifield reported the method of synthesizing Leu-ala-Gly-Val with solid resin as carrier, which marked the beginning of the solid-phase synthesis of peptides and promoted the large-scale production of solid-phase synthesis of peptides. Nowadays, solid - phase synthesis of peptides has been widely used.In the process of peptide synthesis and transportation, it is easy to form related structural impurities, such as amino acid deletion, amino acid insertion, deamination, degradation products, etc. Therefore, the European Pharmacopoeia provides that the content of the structural impurities above 0.5% should be qualitatively analyzed, and the content of the structural impurities above 1% should be quantitatively analyzed and their toxic and side effects should be investigated
The categories of impurities
During synthesis and transportation process, related structure impurities such as amino acid deletion, amino acid insertion, incomplete removal of protecting groups, oxidation/reduction side chain modification, terminal modification, aggregation, etc.
Amino acid deletion
In solid phase synthesis of peptides, the incomplete removal of amino acid protection groups attached to the resin or the introduction of incomplete activation of amino acids reduce the condensation reaction efficiency, resulting in the loss of one or more amino acid residues throughout the peptide chain. During transportation and storage, a small amount of degradation products are also produced, and one or more amino acid residues are missing at the N or C ends, which can also be classified as the impurities with the absence of amino acids.
Amino acid insertion
In solid phase synthesis of peptides, excessive amino acid protection is often added to ensure maximum condensation efficiency.However, if the excess reactant is not completely washed away after the condensation reaction, additional amino acids are inserted into the target peptide sequence.
Oxidation/reduction
In solid phase synthesis of peptides, some amino acid residues are prone to take oxidation/reduction reactions.Impurities formed by oxidation of cysteine and methionine were found in crude products of capitoxin and eletoxin, respectively. comparing with traget peptide, the molecular weight of these impurities increases by 16, 32, and 48 ,depending on the degree of oxidation. ,which are sulfoxide, sulfoxide, and sulfonic acid, respectively.
Side chain modifies impurities
Two basic amino acid side chains, including asparagine and glutamine, contain amino groups. During the synthesis and storage of peptides, the amino group is easy to be lost and the hydroxyl group is added, thus forming the product of deamination. As -CONH 2 is converted to -COOH, the molecular weight of the deaminated product is increased by 1.(The reaction mechanism is shown below)
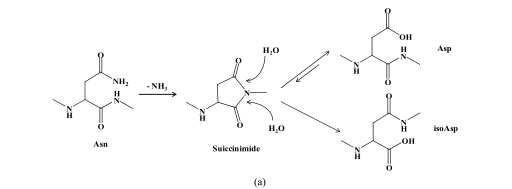
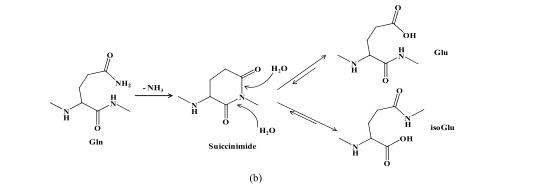
(a) Mechanism of asparagine deamidation; (b) mechanism of glutamine
End-modified impurity
The peptides with glutamine at the N-terminal were apt to be dehydrated and when the temperature was higher than 4˚C during the storage process. In addition, N-terminal acetylation is a common terminal modification.
Aggregation
Peptide aggregation can be divided into covalent and non-covalent types, the degree of formation of which depends on various environmental factors.Covalent aggregates usually consist of two monomers formed by amide bond and disulfide bond.Non-covalent aggregate is the result of weak interaction such as hydrophobic interaction and electrostatic interaction. Usually there is an equilibrium transition between non-covalent aggregates and monomers.This equilibrium is influenced by sample concentration and solvent properties such as pH.
Conclusion
During the synthesis, storage and transportation of peptide drugs, many kinds of structural impurities are produced.The presence of structural impurities in peptide drugs can affect their biological function and pharmacodynamics.Therefore, the analysis and control of structural impurities is one of the important factors to be considered during drug development and clinical use. It is reported in the existing literature that the impurity analysis in peptide drugs usually USES the change of molecular weight to infer the possible amino acid sequence and modification, but there are few studies on the modification sites such as amino acid insertion and deamination.Therefore, it is very important to develop accurate analysis methods for the sequence and modification of impurities in peptide drugs. The development of tandem mass spectrometry and high resolution mass spectrometry will play an important role in the field of impurity analysis in peptide drugs.
Copyright © 2020 Omizzur Inc | Terms & Conditions | Privacy Notice | Sitemap