Fmoc-L-Arg(Pbf)-OH
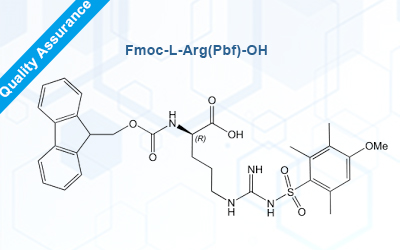
| Name | Fmoc-L-Arg(Pbf)-OH | |
| Type | Fmoc-amino acids | |
| CAS | 154445-77-9 | |
| Molecular Formula | C34H40N4O7S | |
| Pack size | Price ( USD ) | Inventory |
| 100g | $90.0 | In stock |
| 1kg | $800.0 | In stock |

Omizzur Biotech Online Inquiry Form
Notice:
1.Omizzur follows strict guidelines on the use of private information.
2.For bulk inquiry or custom packaging: [email protected]
3.Our customer service staff will contact you in 1 hour. Please check your email

General Description
Synthesis of Peptides
With the development of biotechnology, the use of peptides in the field of biomedicine is becoming increasingly important, and protecting amino acids is the most basic raw material for solid-phase peptide synthesis technology. Amino acids all contain α- Amino and carboxyl groups, some of the 20 amino acids also contain side chain active groups, such as hydroxyl, amino, guanidine, and heterocyclic groups.
Peptide synthesis cannot be separated from the protection of amino acids, and the different protection groups of amino acids have a significant impact on the efficiency and yield of peptide synthesis. Both amino and side chain active groups need to be protected during the reaction. After synthesizing peptides, the protective group is removed, otherwise amino acid mismatches and many side reactions may occur.
According to the activity of side chains during peptide synthesis, 20 amino acids in nature can be divided into inert side chain amino acids (such as Gly, Leu, Ile, Ala, Val, and Phe) and active side chain amino acids (such as side chain carboxyl, side chain amino, side chain thiol, and side chain heterocyclic groups).
The first type only needs protection α- Amino acid, the latter amino acid, also needs to protect the active side chain groups. Due to the removal of the main chain protection group during peptide grafting and the side chain protection group after peptide grafting, these two types of protection groups must have different removal conditions.
Protection/deprotection of amino acids
Different amino acids require suitable protective groups, and the selection of protective groups varies depending on the type of peptide synthesized. Firstly, it is necessary to ensure selective protection of the groups that need to be protected under high reaction yields, and the protected amino acids must have a certain degree of stability. Secondly, the protective group is selectively removed in high yield after the peptide grafting reaction, without affecting the generated peptide bonds. The development of protective groups that can be removed under mild conditions is relatively rapid now.
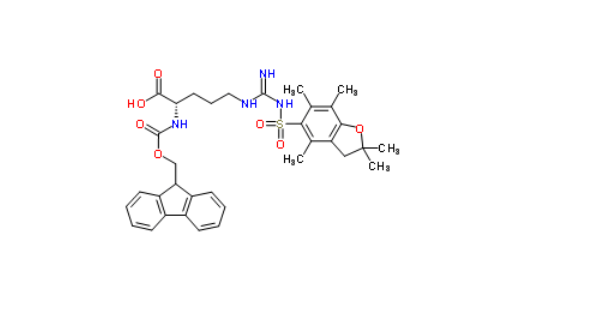
(Fmoc-L-Arg(Pbf)-OH)
Fmoc group: fluorene methoxycarbonyl (Fmoc)
Fluorenemethoxycarbonyl (Fmoc) is an alkali sensitive protective group that can be removed in concentrated ammonia water or dioxane methanol 4N NaOH (30:9:1), as well as 50% dichloromethane solution of ammonia such as piperidine, ethanolamine, cyclohexylamine, 1,4-dioxane, and pyrrolidone.
Under weakly alkaline conditions such as sodium carbonate or sodium bicarbonate, Fmoc protective groups are generally introduced using Fmoc Cl or Fmoc OSu. Compared to Fmoc-C1, Fmoc-OSu is easier to control reaction conditions and has fewer side reactions.
Under acidic conditions, Fmoe protective groups are particularly stable, while they are very sensitive to alkaline conditions. Therefore, they are usually used together with acid sensitive protective groups Boc or Z to protect amino acids containing active side chain groups.
Detection of Fmoc groups
Fmoc has strong ultraviolet absorption, with a maximum absorption wavelength of 267nm( ε 18950), 290nm( ε 5280), 301nm( ε 6200), therefore, UV absorption can be used for detection, bringing many conveniences to the automatic peptide synthesis of the instrument. Furthermore, it is compatible with a wide range of solvents and reagents, has high mechanical stability, and can be used with various carriers and activation methods.
Therefore, the most commonly used in peptide synthesis today is the Fmoc protective group. There are also some alkali sensitive protective groups, such as methylthiomethoxycarbonyl (Mtoc), methylsulfonyl ethoxycarbonyl (Msc), etc.
Omizzur Biotech
Fmoc L-Arg (Pbf) - OH is one of the most commonly used Fmoc protected amino acids in peptide synthesis. Omizzur Biotech is currently available in stock and comes with a complete quality inspection report and certificate. For peptide synthesis click here: custom peptide synthesis
Product Data Sheet
Name | Fmoc-L-Arg(Pbf)-OH |
CAS | 154445-77-9 |
| Alias | Fmoc L Arg Pbf OH |
Type | Fmoc-amino acids |
Molecular Formula | C34H40N4O7S |
Molecular Weight | 648.77 |
Appearance | White to off-white powder |
Optical Rotation (25/D) | -5.5±1.5o (c=1, DMF) |
Diisopropylether Content | 6.5% |
Water Content (KF) | 0.87% |
Solubility | Clear colorless solution (5% m/V in DMF) |
Shipping & Storage
Shipping: Deliver to worldwide by Air / Fedex / DHL
Provide with the goods: Quality analysis report (COA,MS,HPLC) ,TSE/BSE statement (if necessary)
Ordering Guide:
Customer Service Center
 Quote Request :
Quote Request :
* Please mail us your product name, sequences / structural formula, CAS (if any), Omizzur customer services will get in touch with you within 1 hour.
Customer also viewed
 2-Chlorotrityl Chloride Resin
2-Chlorotrityl Chloride Resin
CAS:934816-82-7
 DSIP Peptide
DSIP Peptide
CAS:62568-57-4
Enquiry & Ordering Form
Method 1
Simply email to [email protected] ,you will get a fast response within 1 hour.
Method 2
Submit the simple form to get the latest quotation. Notice:Omizzur follows strict guidelines on the use of private information.
Copyright © 2020 Omizzur Inc | Terms & Conditions | Privacy Notice | Sitemap