Cetrorelix impurities
Peptide drugs are composed of different amino acids in a certain order, and according to the process characteristics of these drugs, there are many synthesis steps that need to be followed, resulting in complex impurities.
What are Peptide Impurities?
The main substances related to the synthesis of peptides are: process impurities introduced during the synthesis process, such as missing peptides, broken peptides, oxidized peptides, and other peptide related substances; Degradation products and polymers produced due to unstable factors such as peptide deamidation, oxidation, hydrolysis, disulfide bond mismatch, racemization, etc.
Peptide Injection Impurities
Peptide drug formulations are mainly injection and injection powder injections, which are more prone to degradation changes and the generation of degradation impurities during production and storage.
In the synthesis of peptides, due to the numerous impurities, complex components, and different properties, some are very similar to peptide drugs, while others have significant differences. Moreover, such impurities may produce significant toxicity at lower concentrations. According to relevant research reports, even if only one or two amino acid sequences in peptides change, they may turn agonists into antagonists and have opposite effects.
Due to the fact that the safety of drugs is not only the toxicity of the main active ingredients, but also the toxicity of impurities, establishing appropriate methods to detect these impurities for substance analysis is the key to quality control of peptide drugs.
What is Cetrorelix ?
Cetrorelix is an LHRH antagonist (artificially synthesized peptides) that can control ovarian stimulation and prevent premature ovulation of immature follicles in clinical practice, helping to conceive.
Cetrorelix studies have shown that cetrexed has good therapeutic effects on diseases such as ovarian cancer, prostate cancer, uterine fibroids, and endometriosis, and has preventive and improvement effects on benign prostatic hypertrophy and ovarian hyperstimulation syndrome.
Cetrorelix impurities play an important role in peptide in-depth research, peptide characterization, and peptide analysis. You can inquiry to Omizzur services for help.
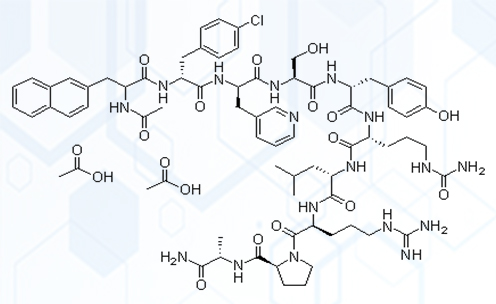
(Cetrorelix structural formula)
European Pharmacopoeia Peptide Impurity Limits
The limits of substances related to peptide drugs have been defined in the Appendix "Substances for pharmaceutical use" of the European Pharmacopoeia 7.0, with reporting limits (0.1%), identification limits (0.5%), and quality control limits (1.0%) established respectively.
3 Detection Methods for Commonly Used Peptide Drug Related Substances
1. Reversed phase high-performance liquid chromatography
High performance liquid chromatography has become the most commonly used method in drug quality analysis, with the characteristics of sensitivity, accuracy, and speed.
Peptide substances are composed of multiple amino acids connected by peptide bonds, with certain hydrophobicity and retention on C18 columns. They can be effectively separated in reverse phase high-performance liquid chromatography. Currently, the analysis of related substances in peptide drugs mostly uses high-performance liquid chromatography.
Due to the complexity of the related substances that may be present in the synthesis of peptide drugs, gradient elution methods are often used, with very few using isocratic elution methods, such as Gonarellin Acetate in EP7.0, Desmopressin in USP34, Leuprorelin, etc.
2. Capillary electrophoresis
In addition to using traditional high-performance liquid chromatography for the analysis of related substances, capillary electrophoresis also has significant advantages for the analysis of peptide drugs. Capillary electrophoresis combines the advantages of high-pressure electrophoresis and high-performance liquid chromatography, and differs from the hydrophobic separation mechanism of high-performance liquid chromatography. There are multiple separation modes, with characteristics such as fast analysis speed, high resolution, low sample dosage, simple operation, and low consumption.
The application of capillary electrophoresis in pharmacopoeia of various countries is also increasing. USP, BP, and Chp all include capillary electrophoresis method, and provide a detailed introduction to its definition, classification, basic principles, instrument structure, and operating parameters. In USP and BP, capillary electrophoresis has been used in the quality standards of multiple drug varieties, which has great advantages in impurity analysis, especially in peptide protein drug impurity analysis.
At present, there are relatively few quality control methods for peptide drugs using capillary electrophoresis as the detection method. In European Pharmacopoeia 7.0, the determination of substances related to glutathione is carried out using capillary electrophoresis.
With the continuous improvement of capillary electrophoresis methods and the development of instruments, overcoming the shortcomings of low reproducibility and precision, capillary electrophoresis (CE) will gradually become a powerful tool for peptide drug analysis.
3. High performance liquid chromatography-mass spectrometry
Liquid chromatography-mass spectrometry technology has become an important means of separating and identifying various compounds. MS is a powerful structural analysis tool that can provide more information for structural characterization. As an ideal chromatographic detector, it not only has strong specificity, but also has extremely high detection sensitivity.
Since the emergence of "soft ionization" technology, it has been possible to analyze highly polar, difficult to volatilize, and unstable biological macromolecules. With the development of biological mass spectrometry, HPLC-ESI-MS/MS is now widely used in the separation and analysis of biological macromolecules such as peptide proteins, for molecular weight measurement and structural analysis.
When using liquid chromatography-mass spectrometry for the analysis of peptide related substances, the first or even multi-level mass spectrometry information of impurities can be provided without obtaining pure impurities, thereby obtaining the molecular weight and sequence information of the impurities, providing a basis for the qualitative and structural analysis of related peptides.

Copyright © 2020 Omizzur Inc | Terms & Conditions | Privacy Notice | Sitemap